Exploring the Hidden Facts About Biosimilar Medications
This article provides an in-depth look at biosimilar medicines, their differences from biologics, usage in psoriasis treatment, and potential side effects. It emphasizes the importance of professional medical consultation and ongoing research in this field, offering valuable insights for patients and healthcare providers alike.

Exploring the Hidden Facts About Biosimilar Medications
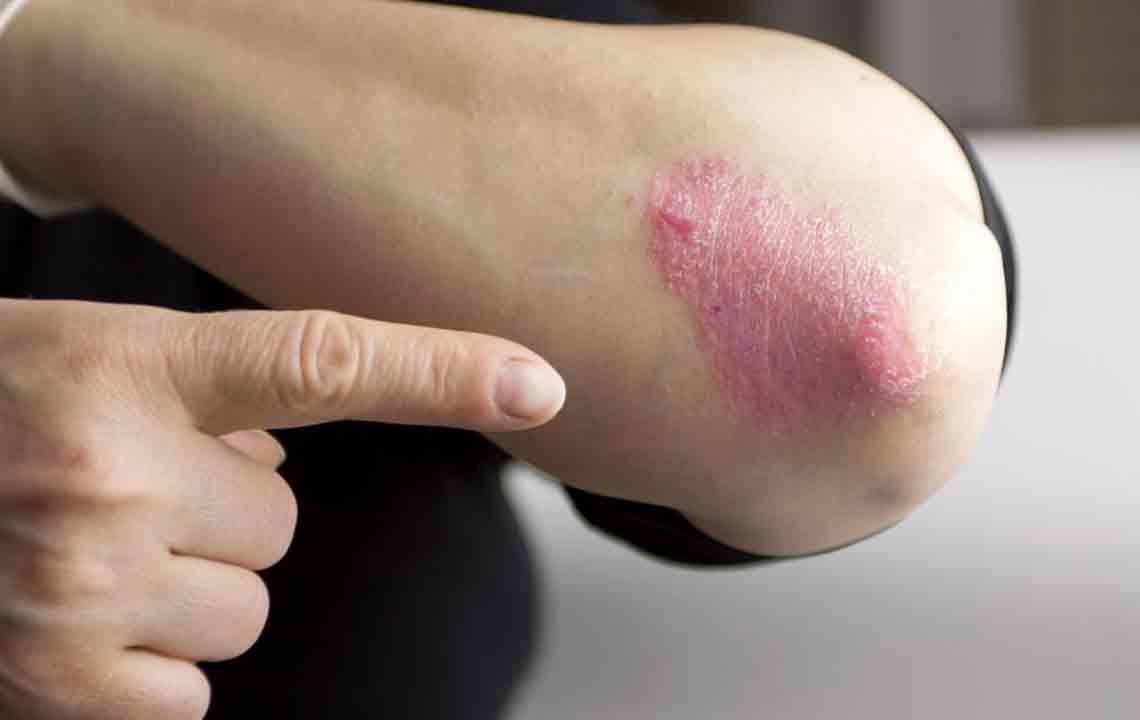
Psoriasis has become a significant health issue in the United States, with various types such as plaque, inverse, guttate, and erythrodermic psoriasis. This condition can spread to different parts of the body, including the nails. The National Psoriasis Foundation supports the use of certain biosimilar drugs as part of treatment options.
Are Biologics and Biosimilars Different?
Biologic therapies target specific components of the immune system, minimizing broad effects on the entire body. Biosimilars closely resemble these biologics but have different safety profiles and regulatory considerations.
Furthermore, biosimilars typically have similar mechanisms of action and dosing regimens as their biologic counterparts.
Who Can Use Biosimilars?
Biosimilars are mainly prescribed for advanced psoriasis cases but are now also considered for patients with active infections or compromised immune systems. Their safety for use in infants, fetuses, and pregnant women remains under ongoing research, and such medications are only recommended when critically necessary.
Utilization of Biosimilars in Psoriasis Treatment
Multiple biosimilar drugs for psoriasis have received FDA approval, including:
Reflexes and Inflectra, similar to Remicade
Erelzi, akin to Etanercept
Adalimumab-adbm and Adalimumab-atto, comparable to Adalimumab
Potential Side Effects of Biosimilars
Like all medications, biosimilars for psoriasis may cause adverse reactions, including:
Headaches
Skin redness, warmth, swelling, and soreness
Upper respiratory infections
Flu-like symptoms such as high fever and chills
Excessive sweating
Chest and nasal congestion
Shortness of breath
Abdominal pain
Injection site reactions
Researchers continue exploring biosimilars’ potential in treating various diseases beyond psoriasis. However, these medicines should only be administered under the supervision of qualified healthcare professionals at reputable centers like Mayo Clinic or UCSF Medical Center.